Epithelial Research Group
at Newcastle University
Research Profile
Research Strategy
Facilities
Collaborations
Funding
Research Strategy
The specialised roles of epithelial cells in health and disease are explored using an integrative translational approach from genome to whole organism. Our overall goal is to be at the forefront of translational research bridging between genomic/proteomic data to functional characterisation/applied research in nutrition, disease states (cystic fibrosis, renal cyst disease, inflammatory bowel disease, neuroblastoma), microbial-epithelial interactions, and pharmacokinetics.
Research falls into three main areas:
Nutrient, micronutrient and drug transport
Electrolyte movement and control of epithelial microenvironment
Epithelial defence
Nutrient, Micronutrient and Drug Transport
Membrane transporters are the gatekeepers of all cells and organelles controlling influx and efflux of essential organic and inorganic solutes, drugs and excretory products. Mammalian genome mapping projects have revealed a wealth of information predicting the existence of more plasma membrane transport proteins than previously detected functionally. Previously unidentified transport systems will play fundamental roles in organismal biology but their importance has been overlooked and new transport systems are emerging as potential therapeutic targets. The overall aim of these studies is to identify the basic functional characteristics of individual transport systems so that the physiological and pathophysiological roles can be identified and rational approaches to drug design developed. Current research projects include those investigating the function of nutrient and micronutrients transporters (e.g. those transporting amino acids, peptides, divalent metals, monocarboxylates, phosphate and pyrophosphate), drug efflux pumps, and organic cation and anion transporters.
is to identify the basic functional characteristics of individual transport systems so that the physiological and pathophysiological roles can be identified and rational approaches to drug design developed. Current research projects include those investigating the function of nutrient and micronutrients transporters (e.g. those transporting amino acids, peptides, divalent metals, monocarboxylates, phosphate and pyrophosphate), drug efflux pumps, and organic cation and anion transporters.
Dr. Colin Brown
Prof. Dianne Ford
Prof. Barry Hirst
Prof. Nick Simmons
Prof. David Thwaites
Dr. Ruth Valentine
Dr. Andi Werner
Electrolyte Movement and Control of Epithelial Microenvironment
Membrane transporters and ion channels are responsible for electrolyte and fluid movement across epithelia which in turn regulate and maintain the ionic composition within the microdomains bathing both intracellular and extracellular faces of epithelial membranes. Post-genomic research focusses on the physiological function of individual transport proteins and the way these various transport proteins 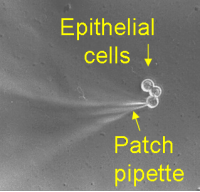 function cooperatively to allow optimal epithelial function. The overall aim of these studies is to define how control of epithelial electrolyte and fluid movement allows optimal epithelial function and how dysfunction leads to various pathophysiological conditions. A better understanding of how these interactions contribute to disease will enhance the development of novel therapeutic approaches. Current research projects include those investigating the function and dysfunction of Cl- channels, anion exchangers, Na+/H+ exchangers, pH regulatory mechanisms and primary cilia.
function cooperatively to allow optimal epithelial function. The overall aim of these studies is to define how control of epithelial electrolyte and fluid movement allows optimal epithelial function and how dysfunction leads to various pathophysiological conditions. A better understanding of how these interactions contribute to disease will enhance the development of novel therapeutic approaches. Current research projects include those investigating the function and dysfunction of Cl- channels, anion exchangers, Na+/H+ exchangers, pH regulatory mechanisms and primary cilia.
Prof. Barry Argent
Dr. Mike Gray
Dr. John Sayer
Prof. Nick Simmons
Prof. David Thwaites
Epithelial Defence
The epithelial layers lining many tissues play vital roles in maintaining barrier integrity by providing protection against endogenous secretions, dietary components and bacterial pathogens. The overall aims of these studies are to identify the role played by mucus secretions in epithelial barrier protection, to determine the nature of epithelial-bacterial interactions and to determine the role played by antimicrobial peptides in host defence.
pathogens. The overall aims of these studies are to identify the role played by mucus secretions in epithelial barrier protection, to determine the nature of epithelial-bacterial interactions and to determine the role played by antimicrobial peptides in host defence.
Dr. Judith Hall
Prof. Barry Hirst
Prof. Jeff Pearson
Prof. Nick Simmons
Dr. Chris Ward
Facilities
The ERG is based in newly-refurbished laboratories within the Faculty of Medical Sciences. We use a broad range of techniques (molecular to whole organism) to allow key questions posed by high throughput technologies in genomics and proteomics to be addressed with respect to organ and whole animal physiology and human studies. The imaging  facility portfolio includes DIC, epifluorescent, photometric and confocal laser scanning microscopy to study intracellular signals (pH, Ca2+), protein and organellar targeting (primary cilium) and transport protein function (in response to changes in nutrient, mechanical and chemical signals and external pathogens). State-of-the-art live cell imaging and electrophysiological techniques (dual-electrode voltage clamp, automated voltage clamp and patch clamp) allow functional measurements from the single protein (either endogenous or heterologously-expressed) to whole tissue making investigation of normal function, as well as the effects of natural polymorphisms and disease-causing mutations possible. An extensive Class II human tissue culture facility (8 laminar flow cabinets and 16 CO2 incubators) was recently re-equipped and is located in SRIF2-refurbished laboratories. Other specialist equipment includes oocyte injection rigs, microtome, real-time PCR and computerised gel analysis systems.
facility portfolio includes DIC, epifluorescent, photometric and confocal laser scanning microscopy to study intracellular signals (pH, Ca2+), protein and organellar targeting (primary cilium) and transport protein function (in response to changes in nutrient, mechanical and chemical signals and external pathogens). State-of-the-art live cell imaging and electrophysiological techniques (dual-electrode voltage clamp, automated voltage clamp and patch clamp) allow functional measurements from the single protein (either endogenous or heterologously-expressed) to whole tissue making investigation of normal function, as well as the effects of natural polymorphisms and disease-causing mutations possible. An extensive Class II human tissue culture facility (8 laminar flow cabinets and 16 CO2 incubators) was recently re-equipped and is located in SRIF2-refurbished laboratories. Other specialist equipment includes oocyte injection rigs, microtome, real-time PCR and computerised gel analysis systems.
Collaborations
We maintain active links with clinical centres in Newcastle, and have international collaborations in North America (Augusta, Chapel Hill, Dallas, Baltimore, Toronto), Europe (Zurich, Antwerp, Munich, Gottingen, Copenhagen, Poitier, Bari, Szeged), UK (Imperial, Mill Hill, Manchester, Dundee, Belfast, East Anglia), and major Pharma (Pfizer, AstraZeneca, GlaxoSmithKline, Novartis, Sanofi-Aventis, Syngenta).
Funding
Research funding includes support from the Biotechnology and Biological Sciences Research Council, Medical Research Council, Wellcome Trust, Food Standard Agency, Royal Society, Cancer Research UK, Kidney Research UK, Northern Counties Kidney Research Fund, Hypertension Trust, Dunhill Medical Trust, GlaxoSmithKline, AstraZeneca, Novartis, Sanofi-Aventis and Syngenta.